Summary
We are interested in the structure, function and regulation of G protein-coupled receptors (GPCRs), their interacting proteins, and psychiatric disease associations such as schizophrenia, suicide and alcoholism. In addition, our laboratory aims to understand the molecular, cellular and neural circuit mechanisms by which environmental factors and chronic drug exposure alter behavior. Our research is based on the combination of interdisciplinary approaches ranging from computer structural modeling and molecular pharmacology in tissue culture to neurochemistry, epigenetics, mouse behavioral assays relevant psychiatric disorders, and functional testing in postmortem human brain. Ultimately, our goal is to use this basic knowledge to develop new approaches for treatment and prevention of psychiatric disorders.
Molecular targets of psychedelics
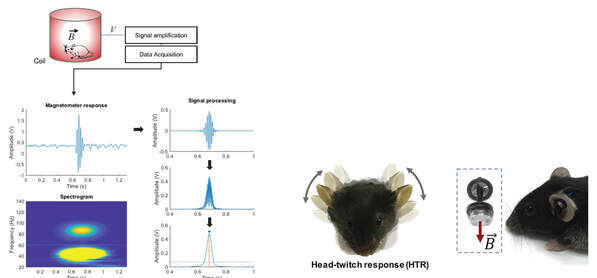
There is a renewed interest on the molecular pharmacology of psychedelics. This focus has been driven, in part, by recent clinical trials suggesting that Schedule I psychedelics, such as psilocybin, mescaline or lysergic acid diethylamide (LSD), may represent a new promising pharmacological strategy to treat depression and other severe psychiatric conditions. As an example, the NIH-NIDA has recently supported the very first clinical study to test psilocybin in the treatment of tobacco use disorder. Although these clinical effects of classical psychedelics are enticing and attractive, one of the most fundamental questions still open in the psychedelic field is whether their hallucinogenic and subjective psychological experience is necessary for their enduring therapeutic effects. Today it is believed, mostly based on our previous work in rodent models, along with findings by other research groups in healthy human volunteers, that the main molecular target responsible for the hallucinogenic effects of psychedelics involves activation of the serotonin 2A (5-HT2A) receptor. We are now investigating the molecular and neural circuit mechanisms underlying the potential clinical use of psychedelics, with the ultimate goal of developing safer, more effective, and non-hallucinogenic treatment strategies.
Structure and function of the 5-HT2A-mGlu2 heteromeric receptor complex
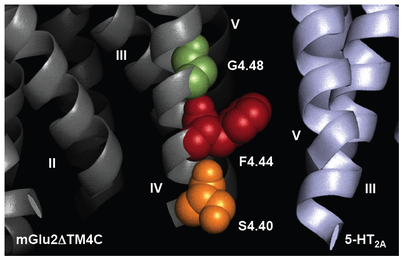
GPCRs have been traditionally considered to exist and function as monomeric structural units that couple to intracellular heterotrimeric G proteins upon ligand binding. Nevertheless, although it remains controversial, multiple points of evidence support the hypothesis that GPCRs also assemble into homomeric and heteromeric complexes. Heteromers of GPCRs are of particular interest because they have been suggested to exhibit specific pharmacological and signaling properties as compared with homomeric GPCRs in vitro in tissue culture. As yet, the functional significance of heteromeric GPCR complexes in whole animal models remains largely unexplored.
Family A serotonin 5-HT2A receptor and family C metabotropic glutamate 2 and 3 receptors (mGlu2/3) are GPCRs that have been linked to the pathophysiology of schizophrenia and other psychotic disorders, as well as to the mechanism of action of atypical antipsychotic drugs (e.g., clozapine, olanzapine and risperidone) and the new class of potential antipsychotic drugs acting as agonists of mGlu2/3 (e.g., LY379268 and LY404039). Notably, although 5-HT2A and mGlu2 receptors belong to different GPCR families, we established the existence of 5-HT2A-mGlu2 heterocomplexes by several biophysical methods. We have compelling data suggesting that this novel 5-HT2A-mGlu2 receptor complex may be responsible for some psychotic symptoms and cognitive deficits in schizophrenia patients, and that it is the direct target of these two different classes of antipsychotic drugs. Our work under this project is focused on the translation of structural findings observed in heterologous expression systems into signaling mechanisms and behavioral events in whole animal models of schizophrenia and antipsychotic drug action. These results are expected to lead to a significantly greater understanding into the structure and function of 5-HT2A and mGlu2 as a GPCR heteromeric complex, and may provide a route to the identification of completely new classes of drugs for schizophrenia treatment.
Family A serotonin 5-HT2A receptor and family C metabotropic glutamate 2 and 3 receptors (mGlu2/3) are GPCRs that have been linked to the pathophysiology of schizophrenia and other psychotic disorders, as well as to the mechanism of action of atypical antipsychotic drugs (e.g., clozapine, olanzapine and risperidone) and the new class of potential antipsychotic drugs acting as agonists of mGlu2/3 (e.g., LY379268 and LY404039). Notably, although 5-HT2A and mGlu2 receptors belong to different GPCR families, we established the existence of 5-HT2A-mGlu2 heterocomplexes by several biophysical methods. We have compelling data suggesting that this novel 5-HT2A-mGlu2 receptor complex may be responsible for some psychotic symptoms and cognitive deficits in schizophrenia patients, and that it is the direct target of these two different classes of antipsychotic drugs. Our work under this project is focused on the translation of structural findings observed in heterologous expression systems into signaling mechanisms and behavioral events in whole animal models of schizophrenia and antipsychotic drug action. These results are expected to lead to a significantly greater understanding into the structure and function of 5-HT2A and mGlu2 as a GPCR heteromeric complex, and may provide a route to the identification of completely new classes of drugs for schizophrenia treatment.
The environment and schizophrenia
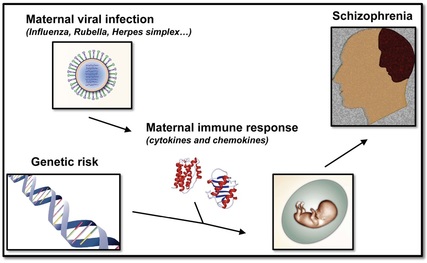
Schizophrenia is one of the most incapacitating mental disorders that affects almost one percent of the world’s population. There is extensive evidence to suggest that genetics plays a significant role in the etiology of schizophrenia. The genetic determinants, however, are complex as clearly elucidated from studies on twins. Monozygotic twins, whose DNA sequences are ~100% identical, have a concordance for schizophrenia of nearly 50%. Such results favor a significant contribution of genetic factors to the etiology of schizophrenia. At the same time, they argue for a significant role of environmental events in the development of this complex psychiatric disease. Epidemiological studies have indicated that maternal infection during pregnancy with a wide variety of agents, including virus (influenza and rubella), bacteria (bronchopheumonia) and protozoa (Toxoplasma gondii) increase the risk of schizophrenia in the adult offspring. Similarly, maternal adverse life events that occurred during pregnancy, such as war, famine and death or illness in a first-degree relative, have been associated with schizophrenia in the adult offspring. Although interesting, the molecular and neural circuit alterations responsible for the neuropsychiatric symptoms induced by maternal adverse life events during pregnancy are as yet not fully understood. Using mouse models of influenza virus infection and variable stress, we are investigating the basic mechanisms by which environmental insults during pregnancy increase the risk of schizophrenia-related phenotypes in the adult offspring.
Epigenetic mechanisms of schizophrenia treatment
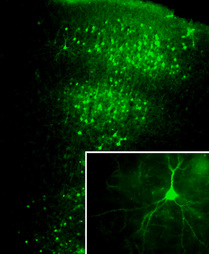
Antipsychotic drugs, including both typical such as haloperidol and atypical such as clozapine and risperidone, remain the current standard for schizophrenia treatment. Nevertheless, about 30% of schizophrenia patients are considered treatment resistant, and will continue to experience psychotic and other symptoms despite the optimal use of antipsychotic medication. Recent preclinical and clinical studies suggest that histone deacetylase (HDAC) inhibitors are efficacious when given chronically in combination with atypical antipsychotics. HDACs remove acetyl groups from lysine residues in the N-terminal tails of core histones, which shifts the balance toward chromatin condensation and thereby silences gene expression. Our ongoing work suggests that a serotonin 5-HT2A receptor-dependent increase in cortical pyramidal HDAC2 promoter activity by chronic treatment with atypical antipsychotics may restrain the therapeutic effects of these agents, and that inhibition of HDAC2 represents a promising new approach to augment the treatment of schizophrenia. Our work is currently focused on the basic molecular and cellular mechanisms involved in the modulation of HDAC2 transcription by chronic treatment with atypical antipsychotic drugs, as well as the role of HDAC2 in mouse frontal cortex as a potential antipsychotic target.
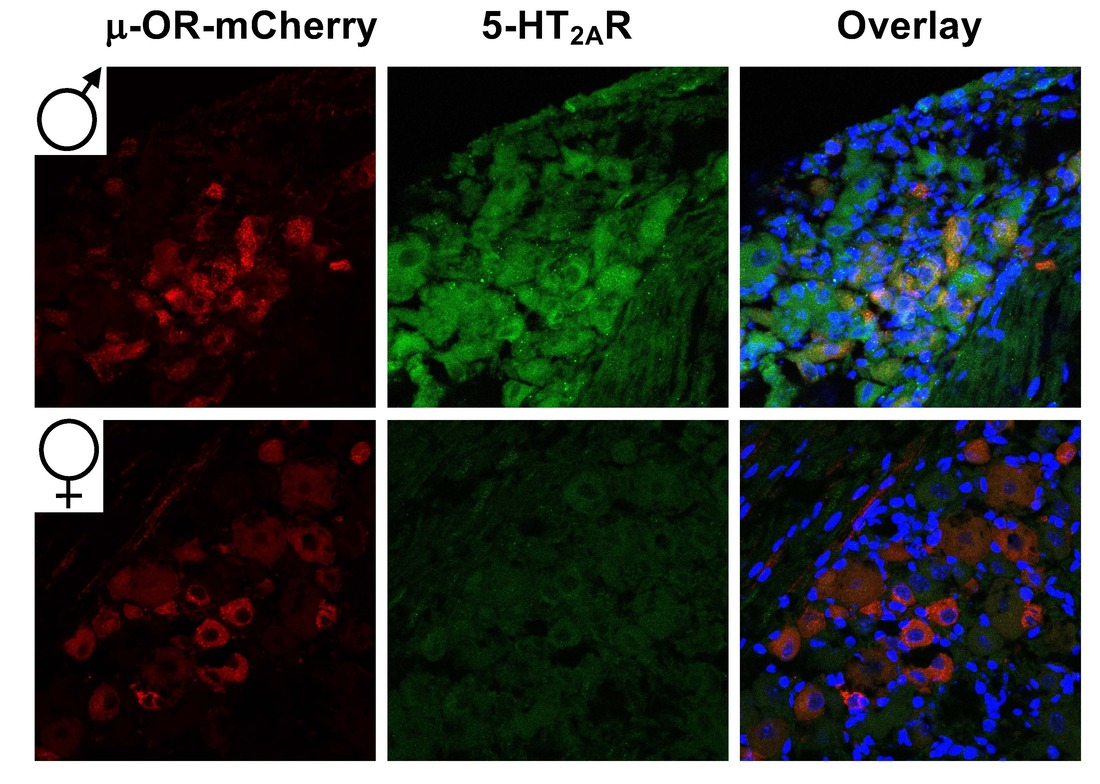
Serotonergic modulation of opioid-induced antinociception
|
Prescription opioid misuse, a serious public health issue in the US, skyrocketed the past decade. However, opioids are still the cornerstone of moderate and severe acute pain management, including post-operative pain and cancer pain. Identification of new pharmacological targets to augment analgesia without increasing the incidence of abuse liability or side effects is therefore a major need in analgesic drug development. Notably, we have recently discovered that lorcaserin, a serotonin 5-HT2CR agonist, augments the antinociceptive effects of opioid analgesics, such as oxycodone, morphine and fentanyl in mouse models. Our current work is focused on investigating the cell signaling and neural circuit mechanism by which 5-HT2CR agonists and 5-HT2AR antagonists behave as adjuvants on opioid-induced antinociception and drug addiction.
|